
![[image]](https://WFMJ.images.worldnow.com/images/18590640_G.jpg)
The Food and Drug Administration has announced the recall of some CVS eye drops over sterility concerns.
Altaire Pharmaceuticals is recalling some over-the-counter drug products sold at CVS Health sold during a specific time period.
The FDA says if the products are not sterile, it could result in serious and potentially life-threatening infections or death.
Altaire says it has not received any reports of adverse effects involving the recalled products.
This recall involves only the specific lots listed below:
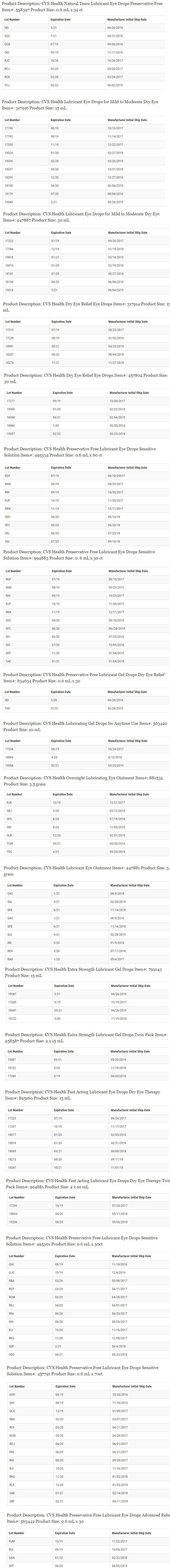
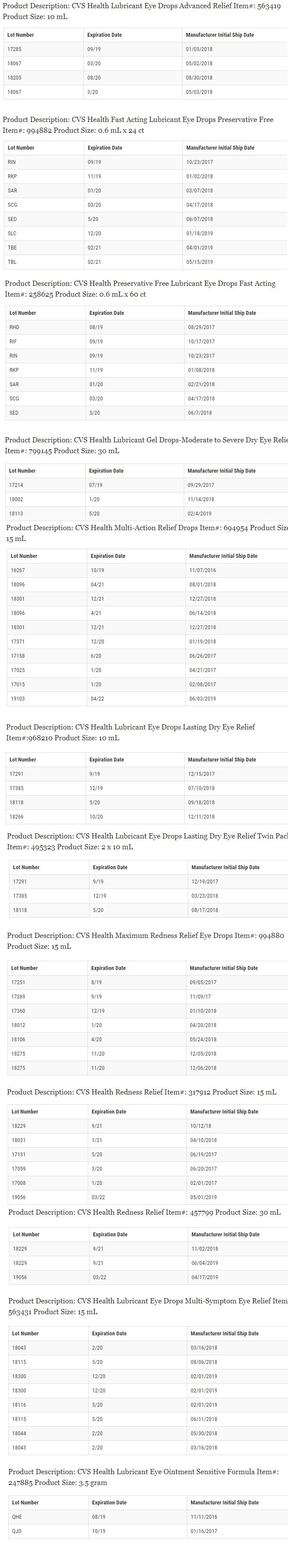
Any questions about the recall can be directed to Altaire Pharmaceuticals Inc., by calling 1-800-258-2471, or e-mailing [email protected] Monday thru Friday from 8:30 a.m. to 5:00 p.m. ET.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using the product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
Complete and submit the report Online
Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178